Discover what the highest rated Respiratory CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Respiratory CRO has to offer.

Discover what the highest rated Dermatology CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Dermatology CRO has to offer.

Discover what the highest rated Psychiatry CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Psychiatry CRO has to offer.

Discover what the highest rated Women's Health CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Women's Health CRO has to offer.

Discover what the highest rated Medical Device CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Medical Device CRO has to offer.

Discover what the highest rated Digital Therapeutics CRO has to offer.
Sep 4, 2025 11:00 AM EDT
Discover what the highest rated Digital Therapeutics CRO has to offer.

Dr. Billings brings over 40 years of experience spanning academia, government, and industry. He has deep expertise in the diagnostics sector, having previously served as Chairman and CEO of Biological Dynamics, a molecular diagnostics company, and as Chief Medical Officer of Natera, a leading genetic testing company focused on oncology, women’s health, and organ health.
Rob is a Director of Strategic Solutions at Lindus Health, overseeing the design and execution of large-scale clinical trials with a focus on diagnostics and screening studies. He has extensive experience in healthcare product development and clinical trial strategy. With a background in behavioral and clinical science, Rob specializes in translating complex operational challenges into scalable, innovative trial designs.
Large-scale liquid biopsy protocols demand tens of thousands of participants, requiring enrollment at many sites within specified timeframes. This often leads to budget and timeline overruns.
"Clinical trials for diagnostics are increasingly time-consuming and expensive, given the large volumes of patients required. Identification and proper enrollment at accredited sites alongside novel methods of reaching patients remotely are promising developments for speeding and focusing efforts." - Dr. Paul Billings
Two approaches have emerged as viable paths forward for large-scale liquid biopsy and multi-cancer early detection (MCED) screening trials: fully decentralized (DCT) models and hybrid designs that run a parallel virtual arm alongside traditional sites. Neither approach is universally superior; the optimal choice depends on the specific protocol, regulatory constraints, and your existing infrastructure.
A DCT model is well-suited when the Schedule of Assessments (SOA) is simple, includes a blood draw and electronic patient-reported outcomes (ePROs), and does not require in-person imaging or diagnostic procedures (see Fig.1). If your eligibility criteria are broad, the addressable participant pool is large and accessible through digital channels such as social media. Participants complete sample collection at local labs through nationwide clinical lab providers like Quest Diagnostics and LabCorp, or via mobile phlebotomy, rather than traveling to a research site. Critically, the protocol doesn't require site-based investigator oversight for every participant interaction.
In this model, enrollment speed is limited only by your recruitment funnel, online advertising spend, and the efficiency of your digital enrollment workflow - not by site capacity.
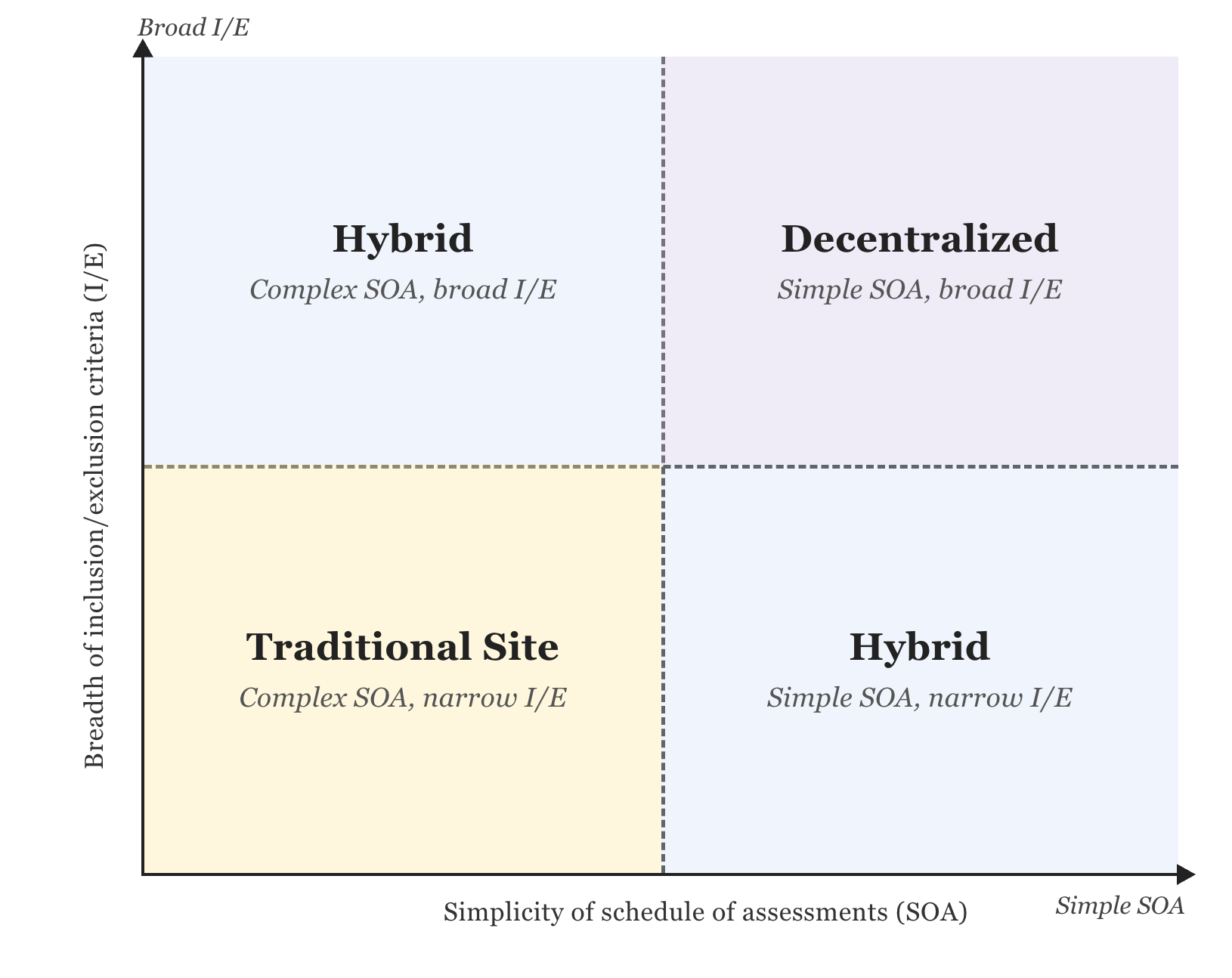
A hybrid model is more suitable when the protocol requires complex assessments after initial screening, such as CT imaging, PET-CT, biopsies, or a specialist diagnostic workup (see Fig. 1). Whether you are planning a new study or currently running a site-based one, a hybrid approach can be tailored to your protocol and integrate & supplement existing sites rather than replacing them.
In both models, the top-of-funnel recruitment strategy looks similar: multi-channel digital advertising across platforms like Facebook, Instagram, Google, Reddit, and TikTok, targeted by demographics and geography. EHR-based searches to identify eligible patients through provider partnerships. Patient community and advocacy group outreach. In a hybrid approach to an existing trial, digital ads can be geo-targeted to direct participants to nearby active site locations.
Sponsors planning trials at this scale may already understand the appeal of DCT and hybrid models, but the operational challenges of implementing these solutions are often less understood, and more importantly, the right solutions to address them. We cover five primary areas in the following sections: identity verification, site engagement, virtual arm data equivalence, human bottlenecks, and sample collection.
With tens of thousands of participants, traditional video-based identity verification creates an enormous bottleneck. Manual video calls at even 15 minutes per participant would require thousands of staff hours, introduce multi-day enrollment delays, and demand weekend and evening staffing. Worse, this bottleneck sits at the exact moment of peak participant engagement, right after someone responds to a digital ad. Every hour of delay increases the probability of participant dropout.
Identity verification is essential for maintaining data integrity, ensuring the ethical obtaining of consent, and adhering to regulatory guidance. Financial incentives can attract fraudulent individuals, and participants may allow others to complete assessments on their behalf. A 2025 Stanford study of a fully decentralized trial found that 62% of screening attempts were fraudulent, and 20% of enrolled participants were later identified as fraudulent due to age and date-of-birth mismatches. At scale, even a small percentage of misidentified participants compromises data integrity and patient safety.
One solution is using automated identity verification integrated into the prescreening flow, allowing participants to complete government-grade authentication in just a few minutes using their smartphone (ID document plus selfie match). No scheduling, with 24/7 availability. At scale, this approach can process tens of thousands of verifications at a nominal cost per verification, compared to the cost of thousands of manual staff hours. This approach can eliminate one of the major sources of top-of-funnel dropout.
Research sites juggle competing studies, and therapeutic trials can generate more revenue and investigator interest than diagnostic screening protocols. Therefore, a screening study can easily slip down a site's priority list.
Overcoming this requires minimizing the enrollment burden on the selected sites. Working with a CRO with access to an extensive network of research sites accelerates activation. For site selection, using data systems, such as Yendou, that provide real-time visibility into each site's current enrollment capacity, historical performance metrics, patient population demographics, and competing trial loads, enables more accurate predictions of which sites will deliver. Equally important is structuring the protocol so that sites aren't burdened with tasks that can be handled virtually by employing online pre-screeners, eConsent, and white-glove scheduling support. This can keep the study manageable for sites while they manage their other commitments.
Sponsors rightfully worry that data collected through a virtual arm won't match the quality or completeness of site-based data.
An effective way to de-risk this is to run a validation pilot early, either before launch or in parallel with site-based enrollment. A pilot should compare structured site EHR extracts with virtual arm data (PDF medical records combined with AI-assisted abstraction, fed directly into an EDC) and demonstrate equivalence. If quality thresholds are met, then proceed to scale the virtual arm.
Traditional enrollment workflows serialize every step through human clinical research coordinator (CRC) capacity: screening, consent, and scheduling. With tens of thousands of participants, CRC bandwidth becomes the rate-limiting factor regardless of how many participants your digital ads are generating.
An alternative is a fully asynchronous digital participant journey. From digital ad to automated pre-screener (with programmatic eligibility logic) to automated identity verification to single-party e-consent to self-scheduled blood draw at a local lab or via mobile phlebotomy. Virtual staff remain available by email and phone, but they don't gate the process. Platforms like CitrusTM, Lindus’ eClinical platform, which has powered over 100 trials, enable this end-to-end digital participant management without requiring app downloads, reducing friction at every step. The entire flow can eliminate participant burden that often accompanies site-based studies.
Even in a fully virtual trial, participants still need to provide a blood sample in person. Recruiting nationwide requires nationwide coverage of collection sites, including areas far from major research centers.
This is addressable through partnerships with national lab networks, such as Quest Diagnostics or LabCorp, for local sample collection, where participants self-schedule at their nearest location, combined with mobile phlebotomy networks. These networks can provide nationwide coverage, including rural areas, closing geographic gaps. For hybrid models, digital advertising can be strategically concentrated in areas with strong overlapping coverage from sites, labs, and imaging vendors to maximize conversion efficiency.
Large-scale screening trials stall when operational infrastructure isn't designed for high volume without escalating costs. Organizations that meet their enrollment timelines align their operational model with protocol requirements and automate steps that don't require human judgment.
Lindus Health has successfully validated this approach across large-scale diagnostic trials, building the infrastructure to support trials with tens of thousands of participants. Our team has worked on some of the largest diagnostic screening trials in oncology, including a 44,000-participant prospective observational study validating blood-based colorectal cancer detection, a 20,000-participant prospective multi-center study for early lung cancer detection, and a 15,000-participant lung cancer liquid biopsy trial. We're supported by leading diagnostics advisors, including Dr. Paul Billings, Ex-Chief Medical Officer of Natera and Chairman of Biological Dynamics, and Professor Bob Langer, Institute Professor at Massachusetts Institute of Technology (MIT) and co-founder of Moderna. We’ve also averaged 85-95% retention rates in past studies with extended long-term follow-ups, powered by our CitrusTM platform and tailored retention strategies.
If you're planning or scaling a large screening trial and want to explore how a DCT or hybrid model could accelerate your enrollment timeline, Lindus can help you determine which model fits your protocol and build the operational plan to execute it.
This is Part 1 of a three-part series on operational strategies for large-scale liquid biopsy screening trials. In Part 2, we'll tackle the challenge of maintaining complete long-term follow-up data across tens of thousands of participants.